 There are more than 750,000 cases of sepsis each year in the United States. The mortality rate of sepsis is 20% and economic cost of sepsis is $17 billion a year. The incidence of sepsis is projected to increase by 1.5% a year. Although the prognosis of sepsis has improved the death rate will increase due to the increased incidence. Sepsis is a killer in the ICU.
There are more than 750,000 cases of sepsis each year in the United States. The mortality rate of sepsis is 20% and economic cost of sepsis is $17 billion a year. The incidence of sepsis is projected to increase by 1.5% a year. Although the prognosis of sepsis has improved the death rate will increase due to the increased incidence. Sepsis is a killer in the ICU.
Sepsis cases are very difficult to litigate. When I review sepsis cases I utilize the components of the Surviving Sepsis Campaign to assess whether the appropriate protocols were implemented during the patients care.
The Surviving Sepsis Campaign was launched in the fall of 2002. It was a collaborative effort of the European Society of Intensive Care Medicine, the International Sepsis Forum, and the Society of Critical Care Medicine. The goal of this campaign was to deduce the mortality rate of sepsis by 25% over the next 5 years. You can view the different aspects of the Surviving Sepsis Campaign at the following URL: www.survivingsepsis.org
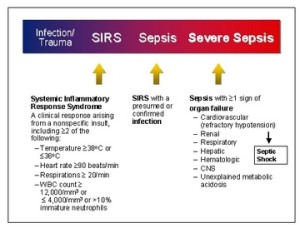
Screening of patients for sepsis is very important. Following are the screening guidelines for sepsis:
Is the patient’s history suggestive of a new infection?
Are any two of following signs & symptoms of infection both present and new to the patient? Note: laboratory values may have been obtained for inpatients but may not be available for outpatients.
Hyperthermia > 38.3 °C (101.0 °F)
Hypothermia < 36 °C (96.8°F)
Altered mental status
Tachycardia > 90 bpm
Tachypnea > 20 bpm
Leukocytosis (WBC count >12,000 µL–1)
Leukopenia (WBC count < 4000 µL–1)
Hyperglycemia (plasma glucose >140 mg/dL) or 7.7 mmol/L in the absence of diabetes
The main parts of the campaign are as follows:
- Fluid resuscitation
- Appropriate cultures prior to antibiotic administration
- Early targeted antibiotic and source control
- Use of vasopressors/inotropes when fluid resuscitation is optimized.
The Surviving Sepsis Campaign implements the use of Bundles. Each hospital may customize their bundles but they must meet standards created by the bundle. Following are the 2 bundles:
TO BE COMPLETED WITHIN 3 HOURS:
1) Measure lactate level
2) Obtain blood cultures prior to administration of antibiotics
3) Administer broad spectrum antibiotics
4) Administer 30 ml/kg crystalloid for hypotension or lactate ≥4mmol/L
TO BE COMPLETED WITHIN 6 HOURS:
5) Apply vasopressors (for hypotension that does not respond to initial fluid resuscitation) to maintain a mean arterial pressure (MAP) ≥65 mm Hg
6) In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥4 mmol/L (36 mg/dL):
–Measure central venous pressure (CVP)*
–Measure central venous oxygen saturation (ScvO2)*
7) Remeasure+ lactate if initial lactate was elevated*
*Targets for quantitative resuscitation included in the guidelines are CVP of ≥8 mm Hg; ScvO2 of ≥70%, and normalization of lactate. (Bundles, 2012)
Recommendations: Initial Resuscitation and Infection Issues:
A. Initial Resuscitation
1. Protocolized, quantitative resuscitation of patients with sepsis-induced tissue
hypoperfusion (defined in this document as hypotension persisting after initial fluid
challenge or blood lactate concentration ≥ 4 mmol/L). Goals during the first 6 hrs. of
resuscitation:
a) Central venous pressure 8–12 mm Hg
b) Mean arterial pressure (MAP) ≥ 65 mm Hg
c) Urine output ≥ 0.5 mL/kg/hr
d) Central venous (superior vena cava) or mixed venous oxygen saturation 70% or 65%,
respectively (grade 1C).
2. In patients with elevated lactate levels targeting resuscitation to normalize lactate
Screening for Sepsis and Performance Improvement
1. Routine screening of potentially infected seriously ill patients for severe sepsis to allow
earlier implementation of therapy (grade 1C).
2. Hospital–based performance improvement efforts in severe sepsis
Diagnosis
1. Cultures as clinically appropriate before antimicrobial therapy if no significant delay
(> 45 mins) in the start of antimicrobial(s) (grade 1C). At least 2 sets of blood cultures
(both aerobic and anaerobic bottles) be obtained before antimicrobial therapy with at least
1 drawn percutaneously and 1 drawn through each vascular access device, unlessthe
device was recently (<48 hrs) inserted (grade 1C).
2. Use of the 1,3 beta-D-glucan assay (grade 2B), mannan and anti-mannan antibody
assays (2C), if available, and invasive candidiasis is in differential diagnosis of cause of
infection.
3. Imaging studies performed promptly to confirm a potential source of infection
Antimicrobial Therapy
1. Administration of effective intravenous antimicrobials within the first hour of
recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C)
as the goal of therapy.
2a. Initial empiric anti-infective therapy of one or more drugs that have activity against
all likely pathogens (bacterial and/or fungal or viral) and that penetrate in adequate
concentrations into tissues presumed to be the source of sepsis (grade 1B).
2b. Antimicrobial regimen should be reassessed daily for potential deescalation (grade
1B).
3. Use of low procalcitonin levels or similar biomarkers to assist the clinician in the
discontinuation of empiric antibiotics in patients who initially appeared septic, but have
no subsequent evidence of infection (grade 2C).
4a. Combination empirical therapy for neutropenic patients with severe sepsis (grade 2B)
and for patients with difficult-to-treat, multidrug-resistant bacterial pathogens such as
Acinetobacter and Pseudomonas spp. (grade 2B). For patients with severe infections
associated with respiratory failure and septic shock, combination therapy with an
extended spectrum beta-lactam and either an aminoglycoside or a fluoroquinolone is for
P. aeruginosa bacteremia (grade 2B). A combination of beta-lactam and macrolide for
patients with septic shock from bacteremic Streptococcus pneumoniae infections (grade
2B).
4b. Empiric combination therapy should not be administered for more than 3–5 days. Deescalation to the most appropriate single therapy should be performed as soon as the
susceptibility profile is known (grade 2B).
5. Duration of therapy typically 7–10 days; longer courses may be appropriate in patients
who have a slow clinical response, undrainable foci of infection, bacteremia with S.
aureus; some fungal and viral infections or immunologic deficiencies, including
neutropenia (grade 2C).
6. Antiviral therapy initiated as early as possible in patients with severe sepsis or septic
shock of viral origin (grade 2C).
7. Antimicrobial agents should not be used in patients with severe inflammatory states
determined to be of noninfectious cause
Source Control
1. A specific anatomical diagnosis ofinfection requiring consideration for emergent
source control be sought and diagnosed or excluded as rapidly as possible, and
intervention be undertaken for source control within the first 12 hr after the diagnosis is
made, if feasible (grade 1C).
2. When infected peripancreatic necrosis is identified as a potential source of infection,
definitive intervention is best delayed until adequate demarcation of viable and nonviable
tissues has occurred (grade 2B).
3. When source control in a severely septic patient is required, the effective intervention
associated with the least physiologic insultshould be used (eg, percutaneous rather than
surgical drainage of an abscess) (UG).
4. If intravascular access devices are a possible source of severe sepsis or septic shock,
they should be removed promptly after other vascular access has been established
Infection Prevention
1a. Selective oral decontamination and selective digestive decontamination should be
introduced and investigated as a method to reduce the incidence of ventilator-associated
pneumonia; this infection control measure can then be instituted in health care
settings and regions where this methodology is found to be effective (grade 2B).
1b. Oral chlorhexidine gluconate be used as a form of oropharyngeal decontamination to
reduce the risk of ventilator-associated pneumonia in ICU patients with severe sepsis (Recommendations: Initial Resuscitation and Infection Issues*, 2012)
I had a very interesting case about a year ago involving a patient that had his septic state missed for a prolonged period of time. He went on to dehisce his wound, have a rupture of one of his anastomosis in his bowel from a recent surgery and have peritonitis. He went on over the next year to have multiple issues stemming from this initial peritonitis and wound up being very debilitated and also with over $1. Million in health care bills. The physician expert that was retained to review this case did not believe in obtaining blood cultures. So even though the patient, early on met the criteria for sepsis, the case was not pursued. Obtaining the correct cultures and getting their sensitivities is a very important part of the Surviving Sepsis Campaign.
We will address other aspects of the Surviving Sepsis Campaign in next week in our Blog post:
Hemodynamic Support and Adjunctive Therapy
Other Supportive Therapy of Severe Sepsis
Works Cited
Bundles. (2012). Retrieved July 25, 2013, from Surviving Sepsis Campaign: http://www.survivingsepsis.org/Bundles/Pages/default.aspx
Recommendations: Initial Resuscitation and Infection Issues*. (2012). Retrieved July 25, 2013, from Surviving Sepsis Campaign: http://www.survivingsepsis.org/Guidelines/Documents/Initial%20Resus%20Table.pdf
Leave a Comment